What Is Used to Describe a Chemical Reaction
Since atoms and molecules can not be seen they have to be imagined and that can be quite difficult. Most often this is written with the format.
To some people chemical equations may seem very hard to understand.

. The difference between chemical and physical changes as it affects chemical reactions. For example the reaction between the aluminium with the metal oxides where aluminium acts as a reducing agent. The chemical energy in a substance is a type of potential energy stored.
The thermite reaction is an exothermic reaction between metal and metal oxide. The type of mechanism is determined based on the chemical property of the monomer and initiator. In a physical process atoms stay where they are.
Genetic material that contains the information that determines inherited characteristics is a molecule called deoxyribonucleic acid DNA. The chemical adhesion of atoms ions or molecules of one substance either a gas liquid or dissolved solid to the surface of another substance resulting in a film of the first substance being weakly bonded to the interface between the two substances. What term is used to describe the link between anabolic and catabolic reactions where intermediate metabolites can move between the reactions.
Chemical reactions are used to make new materials. A chemical reaction is a process in which one or more substances are converted to one or more different substances. All chemical reactions can be represented by equations and models.
A chemical reaction is a process where the arrangement of atoms the way they are connected together is changed. Be able to compare the mass of a. They are to sort cards that show a word description a photograph a word equation or a symbol equation related to a chemical reaction using the following headings.
Use instantaneous rate to describe a chemical reaction. Chemical Energy Describe the role of energy in. If youre seeing this message it means were having trouble loading external resources on our website.
Prove that mass is conserved in chemical reactions. 100k 080 060 H2O2 057 M Slope -00280 molLmin 40 20 30 time min 1 of 4 The instantaneous rate is displayed when you move the cursor over the plot. Chemical reactions are important in biological systems.
Typically the presence of enzymes ____ the activation energy for a reaction to proceed. Chemical Kinetics Chemical kinetics or reaction kinetic is the scientific study of the rates of chemical reactionsThis includes the development of mathematical model to describe the rate of reaction and an analysis of. Understand chemical reactions and the law of conservation of mass.
This is distinct from other changes such as evaporation melting boiling freezing and mixing where changes involve no new substances. Explain its use and give equation. Learn how to use a scale to measure mass.
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. The aluminium reduces the metal oxide most probably an iron lll oxide to produce ferrous and aluminium oxide. B What assumptions were they making when they made this reaction.
The different phases of matter the state symbols and the different terms for them. Passing genetic material to offspring. Substances are either chemical elements or compounds.
C Why do enzymologists only look at the initial. This list shows the most common arrows and their meanings. For example solid ice can.
A chemical reaction is a process in which one or more substances also called reactants are converted to one or more different substances known as products. What term is used to describe the integration of catabolic reactions with anabolic. Chemical reactions involve interaction between chemicals such that all reactants are changed into new materials.
When silver metal is exposed to sulfur it reacts to form silver sulfide. The energy released from the reaction of elements or compounds is called. You can describe a chemical reaction by writing a word equation.
Chemical reaction formulas show the process of how one thing becomes another. Chemical reactions and how they break and form bonds between atoms. The properties of the products are.
In the reaction the atoms of the starting substances are rearranged forming new substances that have different properties. The properties of the new materials are different from those of the reactants. A What is the simplified chemical reaction used by Michaelis and Menten to describe an enzymatically-catalyzed reaction.
01 of 09 Right Arrow. Identify the signs of a chemical reaction. Chemical reactions can be used as energy sources.
Reactant Products Occasionally you will see reaction formulas containing other types of arrows. Balanced reactions reversibility and equlibrium. These reactions can be carried out using anionic mechanisms cationic mechanisms free radical mechanisms and coordination mechanisms.
What is the rate at the very start of the reaction. An enzyme promotes a chemical reaction by serving as a physical site upon which _____ can be positioned for various interactions. Single-celled organisms can reproduce when one cell divides into.
What term is used to describe the minimum amount of energy required for a reaction to proceed. In a chemical reaction the bonds between atoms and molecules get re-arranged breaking some and creating other new ones. Silver sulfide is commonly known as tarnish and turns the surface of silver objects dark and streaky black see Figure below.
The terms used to describe phase changes. A chemical reaction is a rearrangement of atoms in which one or more compounds are changed into new compounds. The monomers that are used for chain polymerization usually contain double bonds triple bonds or aromatic rings.
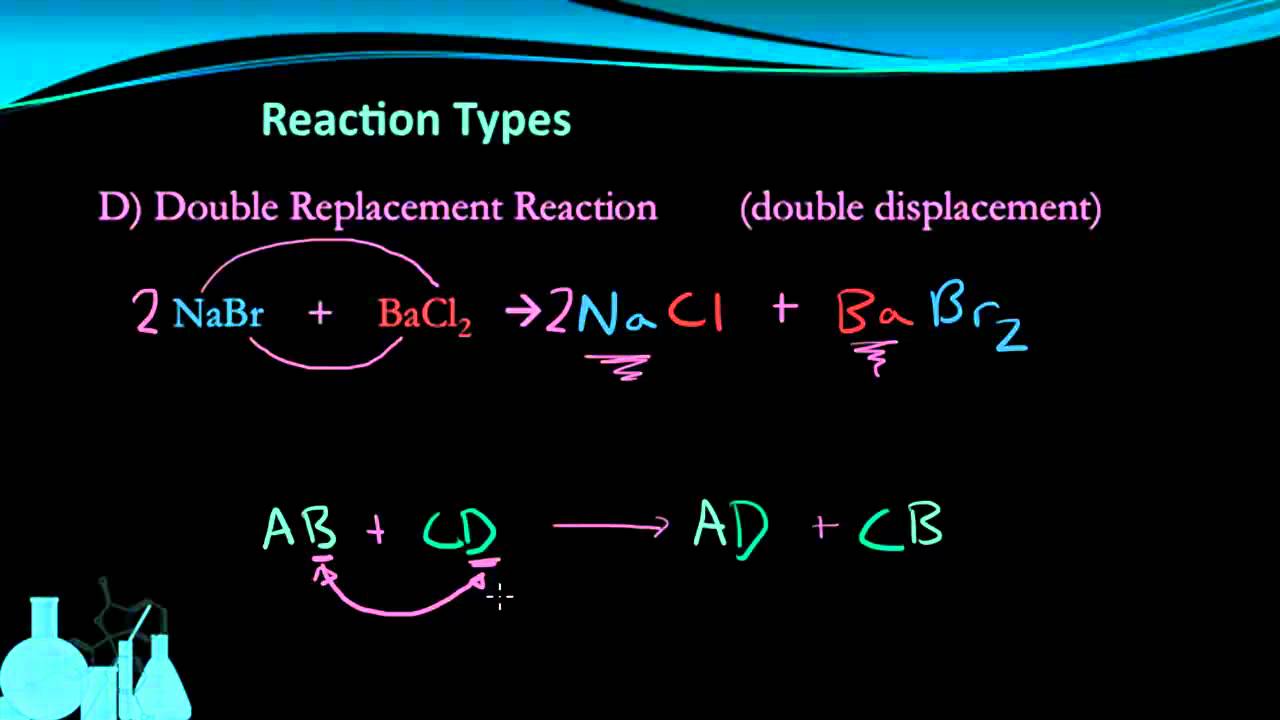
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single

What S Molecular Docking Molecular Docking Is A Computer Tool Used To Simulate Interactions Between Molecules This Is In 2021 Molecular Molecules Chemical Reactions

Chemical Reactions Types Definitions And Examples Teaching Chemistry Chemistry Classroom Chemistry Notes

Chemical Kinetics Reaction Rate The Change In Concentration Of Reactant Or Product Per Unit Time Chemical Kinetics Reaction Rate Gas Constant

Difference Between Empirical And Molecular Formula Infographic Chemistry Lessons Chemistry Study Guide Chemistry Education

Self Assembly Illustration Eco Fashion Nanotechnology Fashion Encyclopedia

Nuclear Chemistry Notes Chemistry Notes Chemistry Lessons Study Chemistry

Basic Chemistry Chemistry Basics Chemistry Classroom Teaching Chemistry

Ms Ps1 5 Conservation Of Matter A Visual Representation Of A Chemical Reaction Chemical Reactions Chemical Visual Representation

Balancing Chemical Equations Worksheet Pdf Digital Chemical Equation Equations Chemistry Activities

Chemical Reactions Worksheets In 2022 Chemical Reactions Science Worksheets 8th Grade Science

Chemical Reactions Poster Teaching Chemistry Chemical Reactions Matter Science

Review Chemical Reactions Worksheet Quiz Chemical Reactions How To Memorize Things Persuasive Writing Prompts

1 Of 54 C Boardworks Ltd 2007 2 Of 54 C Boardworks Ltd 2007 Additional Science Chemistry High School Science






Comments
Post a Comment